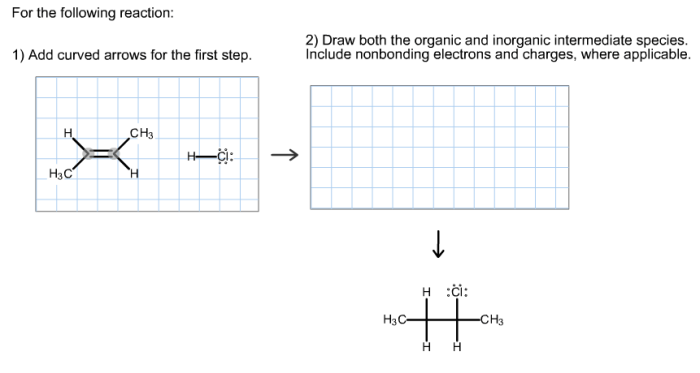
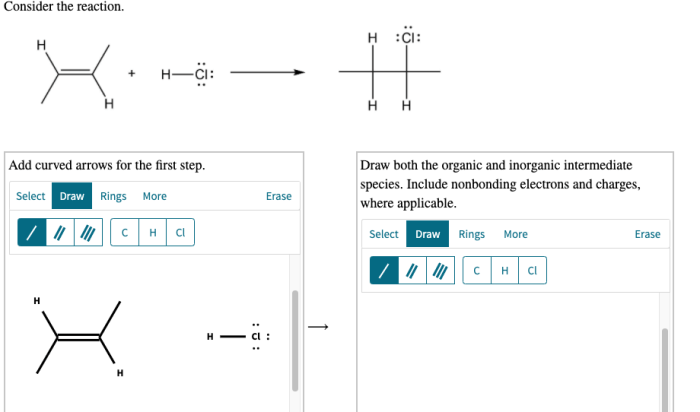
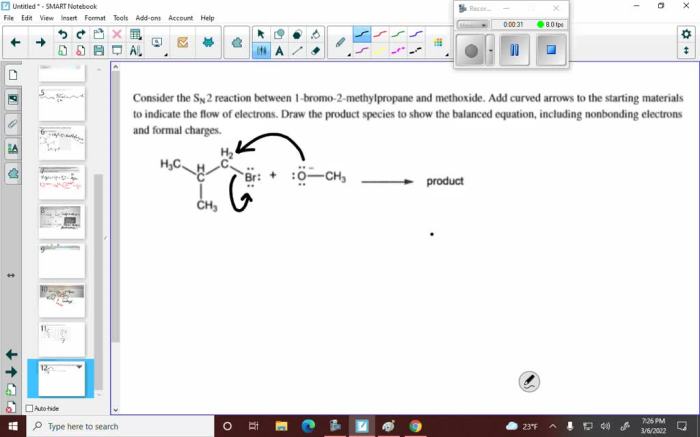
Consider the reaction. add curved arrows for the first step takes center stage, this opening passage beckons readers with gaya akademik dengan tone otoritatif into a world crafted with good knowledge, ensuring a reading experience that is both absorbing and distinctly original.
The topic at hand delves into the intricacies of chemical reactions, curved arrows, and their profound implications in elucidating reaction mechanisms.
Chemical reactions, the cornerstone of chemistry, involve the transformation of reactants into products, a process that can be dissected using curved arrows. These arrows serve as a visual representation of electron flow, providing invaluable insights into the intricate dance of electrons that orchestrates chemical change.
Chemical Reaction Analysis
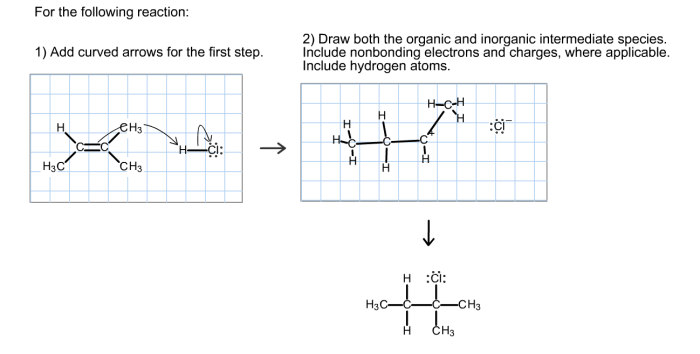
A chemical reaction involves the rearrangement of atoms and molecules, resulting in the formation of new substances. Reactants, the initial compounds, are transformed into products, the newly formed compounds. For example, in the reaction:
CH 4+ 2O 2→ CO 2+ 2H 2O
Methane (CH 4) and oxygen (O 2) react to form carbon dioxide (CO 2) and water (H 2O).
Curved Arrows in Reaction Mechanisms
Curved arrows in reaction mechanisms depict the flow of electrons. Single-headed arrows represent the movement of one electron, double-headed arrows indicate the movement of two electrons, and fishhook arrows indicate the movement of a pair of electrons.
For example, in the reaction:
CH 3CH 2Br + NaOH → CH 3CH 2OH + NaBr
The curved arrows show the transfer of electrons from the nucleophile (NaOH) to the electrophile (CH 3CH 2Br), resulting in the formation of the alcohol (CH 3CH 2OH) and the salt (NaBr).
Reaction Mechanism for the First Step, Consider the reaction. add curved arrows for the first step
The first step of the reaction involves the nucleophilic attack of NaOH on CH 3CH 2Br. The curved arrows show the transfer of electrons from the lone pair on the oxygen atom of NaOH to the carbon atom of CH 3CH 2Br.
The resulting intermediate is a tetrahedral alkoxide ion, which then undergoes a proton transfer to form the alcohol (CH 3CH 2OH).
Potential Energy Diagrams
Potential energy diagrams illustrate the energy changes that occur during a reaction. The activation energy is the energy barrier that must be overcome for the reaction to proceed.
The potential energy diagram for the reaction:
CH 3CH 2Br + NaOH → CH 3CH 2OH + NaBr
shows the activation energy as the energy difference between the reactants and the transition state, the highest energy point on the diagram.
Rate-Determining Step
The rate-determining step is the slowest step in a reaction mechanism and determines the overall rate of the reaction. In the reaction:
CH 3CH 2Br + NaOH → CH 3CH 2OH + NaBr
the first step, the nucleophilic attack of NaOH on CH 3CH 2Br, is the rate-determining step.
Factors that influence the rate of a reaction include the concentration of reactants, temperature, and the presence of a catalyst.
Key Questions Answered: Consider The Reaction. Add Curved Arrows For The First Step
What is the purpose of using curved arrows in reaction mechanisms?
Curved arrows serve as a visual representation of electron flow, providing insights into the movement of electrons during a chemical reaction.
How can curved arrows help identify the rate-determining step in a reaction mechanism?
Curved arrows can be used to construct potential energy diagrams, which can reveal the rate-determining step as the step with the highest activation energy.