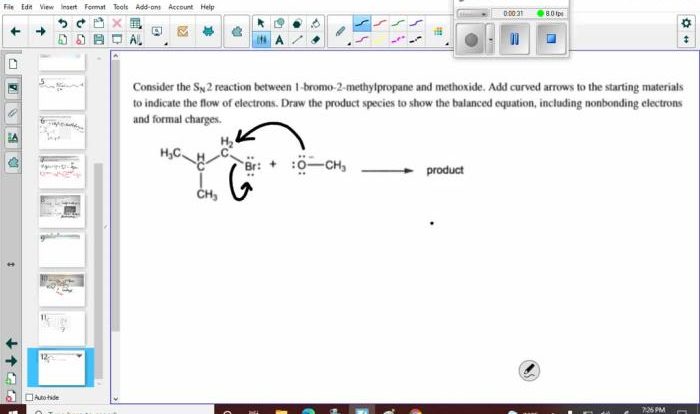
The Basic Atomic Structure Worksheet with Answers is an invaluable resource for students and educators alike, providing a comprehensive overview of the fundamental principles of atomic structure. This worksheet, accompanied by detailed answers, delves into the intricacies of subatomic particles, atomic numbers, mass numbers, isotopes, electron configurations, and the periodic table, empowering learners with a deep understanding of the building blocks of matter.
Through engaging explanations, clear diagrams, and thought-provoking exercises, this worksheet fosters a solid foundation in atomic structure, equipping students with the knowledge and skills necessary for success in chemistry and related fields.
Introduction

Atomic structure is the fundamental arrangement of subatomic particles that make up an atom. It is crucial for understanding the behavior and properties of matter.
A worksheet with answers provides a structured framework for students to explore and reinforce their understanding of atomic structure.
Subatomic Particles
Atoms are composed of three fundamental subatomic particles:
- Protons: Positively charged particles located in the nucleus, with a mass of approximately 1 atomic mass unit (amu).
- Neutrons: Neutral particles located in the nucleus, with a mass slightly greater than that of protons.
- Electrons: Negatively charged particles that orbit the nucleus, with a mass negligible compared to protons and neutrons.
Atomic Number and Mass Number
The atomic number is the number of protons in an atom’s nucleus. It uniquely identifies the element and determines its chemical properties.
The mass number is the total number of protons and neutrons in an atom’s nucleus. It provides an estimate of the atom’s mass.
Isotopes
Isotopes are atoms of the same element that have different numbers of neutrons. They have the same atomic number but different mass numbers.
Isotopes are used in various applications, such as radioactive dating, medical imaging, and nuclear energy.
Electron Configuration
Electron configuration describes the arrangement of electrons in the atomic orbitals around the nucleus.
Rules for writing electron configurations include:
- Aufbau principle: Electrons occupy the lowest energy orbitals first.
- Pauli exclusion principle: No two electrons can have the same set of quantum numbers.
- Hund’s rule: Electrons occupy degenerate orbitals with the same spin before pairing.
Periodic Table
The periodic table is an organized arrangement of elements based on their atomic numbers and electron configurations.
The periodic table allows us to predict the properties of elements based on their position in the table.
Applications of Basic Atomic Structure, Basic atomic structure worksheet with answers
Basic atomic structure has applications in various fields:
- Medicine: Understanding atomic structure is crucial for developing drugs, medical imaging techniques, and radiation therapy.
- Materials Science: Atomic structure governs the properties of materials, enabling the development of new materials with desired properties.
- Energy Production: Nuclear energy relies on the manipulation of atomic structure to generate electricity.
FAQ: Basic Atomic Structure Worksheet With Answers
What is the purpose of the Basic Atomic Structure Worksheet with Answers?
The Basic Atomic Structure Worksheet with Answers is designed to provide a comprehensive overview of the fundamental principles of atomic structure, serving as a valuable resource for students and educators.
What topics are covered in the worksheet?
The worksheet covers a wide range of topics related to atomic structure, including subatomic particles, atomic numbers, mass numbers, isotopes, electron configurations, and the periodic table.
How can I use the worksheet effectively?
The worksheet is best utilized by working through the exercises and questions provided, referring to the answer key for guidance and clarification as needed.
Who can benefit from using the worksheet?
The worksheet is suitable for students and educators seeking to enhance their understanding of atomic structure, particularly those in chemistry and related fields.