Embark on an enriching journey into the realm of chemistry with our empirical and molecular formula worksheet, meticulously crafted to provide a comprehensive understanding of these fundamental concepts. This worksheet delves into the intricacies of determining empirical and molecular formulas, equipping you with the knowledge to decipher the language of chemical compounds.
Delve into the practical applications of empirical and molecular formulas, unraveling their significance in various scientific disciplines and their impact on our daily lives. Prepare to be captivated by the interactive HTML table, where you can witness the transformation of formulas into their corresponding chemical compounds.
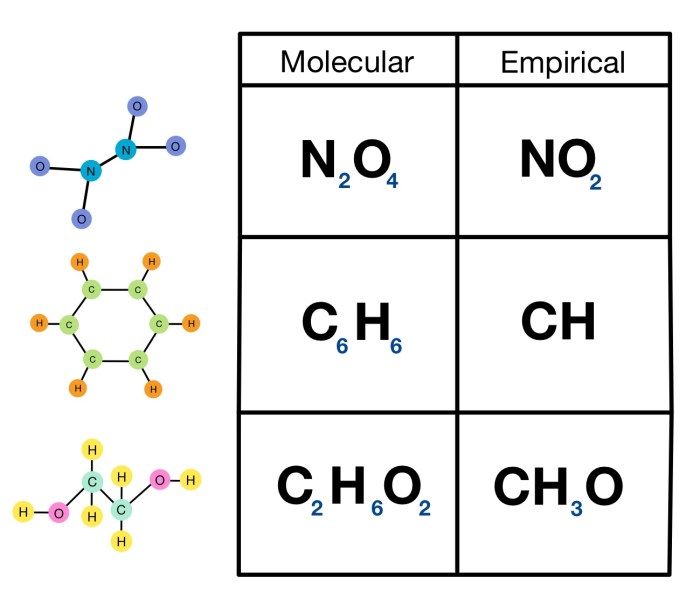
Empirical Formula: Empirical And Molecular Formula Worksheet
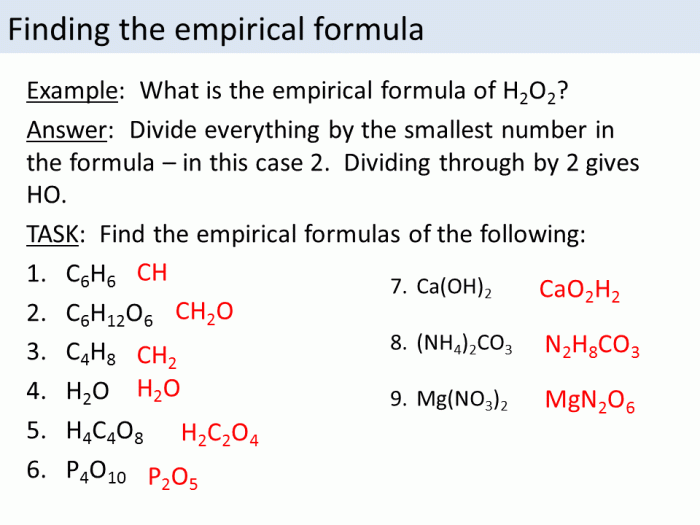
An empirical formula represents the simplest whole-number ratio of the elements present in a compound. It does not provide information about the actual number of atoms or the molecular structure of the compound.
For example, the empirical formula of glucose is CH 2O. This formula indicates that glucose is composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. However, the empirical formula does not specify the molecular formula of glucose, which is C 6H 12O 6.
To determine the empirical formula of a compound, we need to know the mass of each element present in the compound. This information can be obtained through elemental analysis or other analytical techniques.
Molecular Formula
A molecular formula represents the actual number of atoms of each element present in a molecule of a compound. It is a multiple of the empirical formula.
To determine the molecular formula of a compound, we need to know the empirical formula and the molar mass of the compound. The molar mass is the mass of one mole of the compound, expressed in grams per mole (g/mol).
For example, the empirical formula of benzene is CH. The molar mass of benzene is 78.11 g/mol. To determine the molecular formula of benzene, we divide the molar mass by the empirical formula mass:
Molecular formula mass = (12.01 g/mol) + (1.01 g/mol) = 13.02 g/mol
Molecular formula = (78.11 g/mol) / (13.02 g/mol) = C 6H 6
Worksheet Activities, Empirical and molecular formula worksheet
A worksheet with practice problems on determining empirical and molecular formulas can help students to understand these concepts. The worksheet should include a variety of problems with different levels of difficulty.
Here are some sample problems that could be included in the worksheet:
- Determine the empirical formula of a compound that contains 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen.
- Determine the molecular formula of a compound with an empirical formula of CH 2O and a molar mass of 60.0 g/mol.
Real-World Applications
Empirical and molecular formulas have a wide range of applications in chemistry, medicine, and other fields. For example, empirical formulas are used to:
- Identify and classify compounds
- Determine the stoichiometry of reactions
- Calculate the molar mass of compounds
Molecular formulas are used to:
- Represent the structure of compounds
- Predict the properties of compounds
- Design and synthesize new compounds
FAQ Insights
What is the difference between an empirical formula and a molecular formula?
An empirical formula represents the simplest whole-number ratio of elements in a compound, while a molecular formula indicates the actual number of atoms of each element present in a single molecule of the compound.
How can I determine the empirical formula of a compound?
To determine the empirical formula, you need to know the mass of each element present in the compound and then convert these masses to moles. The mole ratios can then be simplified to obtain the simplest whole-number ratio, which represents the empirical formula.
What is the significance of empirical and molecular formulas in real-world applications?
Empirical and molecular formulas play a crucial role in various fields, including medicine, chemistry, and materials science. They are used to determine the composition of substances, predict their properties, and design new materials with specific properties.